In this episode of 7 Minutes of BS, Dr. Joseph Lstiburek, Ph.D., P.Eng., goes long on a BIG topic: the properties, characteristics, and behaviors of water. The ugly part is that it rots houses. It is LITERALLY in its job description. The beautiful part is when water changes phases from one form to another. It's also the dangerous part.
Water is about as simple a molecule as you can imagine
Water (n)
wa·ter | ˈwȯ-tər |
The odorless, tasteless, very slightly compressible liquid that falls as rain and forms streams, lakes, and seas. The major constituent of all living matter. When pure, appears bluish in thick layers. Has a maximum density at 4° C, ionizes to hydrogen and hydroxyl ions, and is a good solvent.
—Edited definition from Merriam Webster
Water is an amazingly interesting thing. H20. Dihydrogen oxide.
It's confused about itself. It is H20 but really wants to be H40, so it's missing two hydrogen molecules, so it's got a big oxygen and two smaller hydrogens stuck off to one side, and it's unbalanced, and it leads to crazy behavior because it's unbalanced.
It is unbalanced because of the electrical charge of Oxygen and Hydrogen. Oxygen has a slight negative charge—it’s always complaining. Hydrogens have a slight positive charge—they’re always looking on the bright side.
Combined, they are kind of shaped like Mickey Mouse—the ears are positively charged, and the chin is negatively charged, making the molecule unbalanced. But it wants to be balanced.
It wants to find two more hydrogens. And so it spends most of its time running around trying to find oxygen to stick to or something.
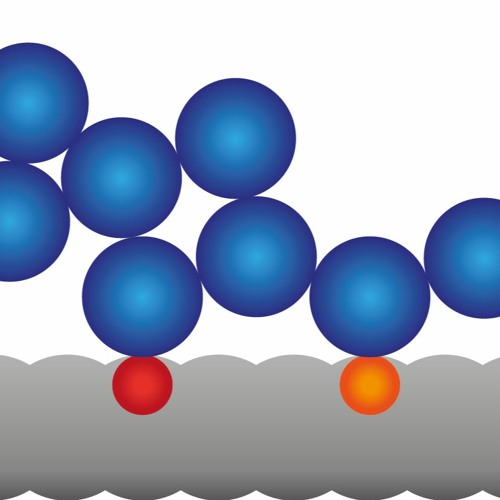
So you've got these tiny water magnets that are running around, sticking to things.
And when something doesn't like them to stick to it, you know, water says, okay, to hell with you, I'm gonna stick to myself.
Materials that water likes to stick to are said to be hygroscopic.
Materials that water doesn't like to stick to are said to be hydrophobic.
You can tell if a material is hygroscopic or hydrophobic by dropping water on it. If the drop flattens out, the water is sticking to the material, and the material is hygroscopic. If the water drop beads up, like that stuff you squirt on your windshield, the material is hydrophobic.
That is one of the simplest and most elegant tests to do.
Thank you, but I didn’t make it up.
Now, here's the problem. You can mess with the characteristics of the water by adding stuff to it. Or you can mess with the characteristics of a surface by adding stuff to it.
Like wiping Rain-X on your windshield.
A classic example would be to put some wax on wood.
Such as a subfloor panel.
And wood normally likes to wick up water. But if you put some wax on it, the water says, ah, I'm not gonna stick to wax. I'm gonna stick to myself.
You can also make water more attractive to hydrophobic surfaces by adding stuff to it. Like soap. Soap is a surfactant, which means that it helps water stick to stuff. People add dish soap to water when they spray stuff on their plants so the solution will spread over the whole leaf instead of beading up, like Rain-X.
So you can change the characteristic of the surface by making it better or worse for water, you can change the characteristic of the water to make it better or worse for the surface.
All of this is relating to something called surface energy, and water wants to minimize its surface energy, and the surface energy of water is 72 Dine centimeters. Well, wood has a surface energy of about 130 dine centimeters.
Whereas wax has a surface energy of 32. So the water says, man, I can't stick to that. I'm gonna stick to myself. So anything below 72 is hydrophobic. Anything above 72 is hygroscopic.
Adding soap to a surface (or to the water you spray at that surface) destroys the water repellency of that surface. This affects houses, too, and surfactants don’t ONLY come in soap bottles. Some of them grow on trees.
There were some famous WRBs. Whose name we can't mention that would lose their water repellency…
…when tannins from the wood siding leached through the siding onto the WRB and changed the surface characteristics of the WRB material, making it permeable to liquid water.
And it took some genius material science to figure out how to change these materials so that they would actually not fail when they put wood siding over them.
However, if we had just used a ventilated rainscreen system, the WRB would never have come into contact with the tannins, and the WRB would never have been improved, so never—oh, wait, ALWAYS use a rainscreen.
For a simple molecule, water is surprisingly complex
Well, one of the most amazing things about water is that when it changes phase from a liquid to a solid, it increases in volume by 8, 9%.
It becomes less dense.
Now you think about that for a moment. Almost all of the other materials on the planet that change from a liquid to a solid increase in density, but water decreases in density.
Well, guess what? That allows life in the Northern Hemisphere to exist.
That’s good news for us mammals, but it's really good news for fish and other aquatic critters. Because ice is less dense than liquid water, lakes, and ponds freeze from the top down. And because water is such a great insulator, the bottom of the lake never gets below about 39 degrees Fahrenheit—even lakes in Siberia only freeze about three feet down.
That's spectacular. That's why icebergs float. Now there's another absolutely freaking amazing thing with water is that when you have certain things in solution with the water,
Like salt
...when the water changes phase from a liquid to a solid, the salt is left behind. That's why icebergs don't have salt in them.
It seems like that would have implications for the durability of stucco brick and concrete.
Now this has enormous implications for the durability of stucco, brick, and concrete. If you have a brick, for example, and the brick begins to get wet, and there's always some soluble salts that turn into a solution.
People say that gee, freeze-thaw happens in bricks because when the water changes phase, it increases in volume and causes the brick to blow up.
That is not at all what happens.
What does happen?
What happens is that as you have saltwater in solution in brick, and a freezing front begins to pass through, as the front begins to freeze, the salt is left behind and increases the concentration of the water that hasn't yet changed phase.
And so you have an increase in salt concentration in the solution.
Water + salt = boatloads of pressure
And water from other parts of the brick rush to that to dilute it. And so these forces are called basically osmotic pressures. Osmosis.
And the pressures are so great that we're talking thousands of pounds per square inch, and the brick blows up. Not all brick blows up. I'm gonna set that aside for a moment.
That's a similar issue with concrete. Concrete is wet and has salts in it, which is quite common. And if the freezing front begins to happen, the water changes phase, osmotic pressures build up and we create spaces for the water in the liquid phase, that's increasing pressure to squirt into, to relieve the pressure.
We call this the "Don Ho Effect."
Don Ho had a major hit record called Tiny Bubbles in 1967
...which was the last year the Toronto Maple Leafs won a Stanley Cup.
And THAT’s where Tim Horton came from
The water would squirt into those pores and relieve the hydrotic pressure. To this day, we use air-entrained concrete. The way we would create air-entrained concrete back in the day is we would add Ivory Snow detergent to concrete, which created basically tiny bubbles.
Now we’re gonna come back to the brick.
Think of brick as a tinker toy. And the tinker toy has got voids in it, and the size of the voids and brick and the tinker toy is based on its firing temperature and its mix.
Some of the brick has sufficient voids, sufficiently distributed, that as a freezing front passes through, it acts like air-entrained concrete.
So some bricks are very resistant to freeze-thaw damage. And then other bricks are not resistant to freeze-thaw damage.
Depending on the brick’s firing temperature. The same concept is true for concrete.
There are concretes that are very resistant to freeze-thaw damage, and there are concretes that are not resistant to freeze-thaw damage.
Now hold that thought.
The key is increasing the concentration of salt in the saltwater solution. One way of doing it, as I just described, is to change some of the liquid to a solid. That increases the concentration of salt, but it hasn't changed phase.
When ocean water freezes, the ice has no salt, so the remaining ocean is saltier.
Another way of doing this is to change the phase of liquid water to a vapor. So what happens is if we have a saltwater solution and then the water evaporates, the salt is left behind, increasing the concentration of the salt in the water that hasn't yet evaporated.
We all did this Petri dish experiment in elementary school, put salty water in a petri dish, and let the water evaporate to reveal the salt left in the petri dish. The same thing happens inside bricks.
That also leads to tremendous. Osmotic forces, and we call that spalling and efluorescence.
And we’ve got a kick-ass podcast episode about that with Sarah Gray.
So think about this, it's the change in phase of water that makes things really weird and interesting. So one change in phase is from a liquid to a solid...
When water freezes
...and another is a change in phase from a liquid to a vapor.
When water evaporates
How to make concentrated wine using physics
And both result in extreme high pressures. Now, another way of explaining it to the youngsters I deal with is that think of this as ice wine (or icewine; German: Eiswein) and Vin Santo.
Wait. What’s ice wine?
Ice wine increases the concentration of the flavor of the grapes because the water in the grapes freezes, and what's left behind is a higher concentration of beautiful nectar. Well, ice wines were pioneered in Germany and exported to Canada, Niagara Falls, and they are awesome.
Well, the Italians didn't have cold winters, so what they would do is they would evaporate the water with the sun. Vin Santo, the wine of the Saints.
And so the idea was I'm changing the phase of the water to improve the performance of my wine.
Of course, with concrete and brick and stucco, I am changing the phase of the water to hurt the performance of my concrete and brick and stucco.
So, again, the mechanisms are, are absolutely precious and unique to water. And the freaking unbalanced H20 polar molecule is absolutely magnificent and magic in terms of not just wines but materials in building science and life on the planet.
Let’s not forget about beer and water skiing.
We want to thank Dr. Joe for jumping in our puddle and remind you that you get paid for what you do and what you know. And now, you know more. 7 Minutes of BS is a production of the SGC Horizon Media Network